- BD Nexiva™ Catheter System
- Clinical Evidence
- Interactive Configurations
- Products and Accessories
- EIFU & Resources
Enhance patient care with
an all-in-one closed IV
catheter system
BD Nexiva™ Closed IV Catheter System is part of a broad portfolio of closed, integrated peripheral IV catheters (PIVCs) designed with the patient and clinician experience in mind.
Enhance patient care with an all-in-one closed IV catheter system
BD Nexiva™ Closed IV Catheter System is part of a broad portfolio of closed, integrated peripheral IV catheters (PIVCs) designed with the patient and clinician experience in mind.
Clinicians prefer closed catheter systems
Overall clinician satisfaction has been shown to be significantly higher with Nexiva™ Catheter System compared to a non-integrated, open PIVC.*,1
The integrated system is designed with clinician safety in mind:
- Clinically demonstrated to reduce the risk of blood exposure by 98%2
- Minimizes risk of contamination with pre-attached extension tube that contains blood upon insertion and serves as a secondary flash chamber to support first-attempt insertion success
- Automatic shielding provides passive safety of needle upon removal as recommended by Infusion Therapy Standards of Practice3
*compared to a non-integrated PIVC in a clinical study
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
See the power of a closed
catheter system
Watch this video to see the differences in design between the integrated Nexiva™ Catheter System and a non-integrated PIVC without blood control technology, which can risk missed insertion, blood leakage, or touch contamination.
Elevate care while helping reduce complications
Complications may compromise patient care and increase cost, including interrupting drug administration, delaying procedures, and removing and reinserting a new catheter, increasing the risk of catheter failure with each subsequent insertion.4–6
Nexiva™ Catheter System has been clinically demonstrated to significantly reduce catheter-related complications in a number of studies:2,7
†BD Nexiva Closed System with 3M™ Tegaderm IV Securement Dressing compared to
B. Braun Introcan Safety® Catheter with Statlock® IV Ultra Stabilization Device and non-bordered dressing.
‡ with a PTFE catheter
A budget-friendly catheter solution
In a 2014 clinical study, the longer dwell time§ of Nexiva™ Catheter System led to a cost reduction of approximately $1 million per year per 1,000 beds compared with an open system.7
Longer median dwell time compared to an open system4,7
Nexiva™ Catheter System eliminates the need to purchase many separate components. Every Nexiva™ Catheter System contains a pre-attached extension tube and built-in stabilization platform. Nexiva™ Catheter System is offered in configurations containing BD MaxZero™ Needle-free Connectors and BD Q-Syte™ Needle-free Connectors.
§compared to an open system
Support 1st attempt insertion success
IV catheter failure rates lie between 35% and 50%. Compounding this issue, subsequent PIVCs inserted after treatment failure are more likely to fail again, potentially perpetuating the challenges and dissatisfaction for the patient.6
Higher first attempt insertion success may be a relief to patients. Studies have shown:
Nexiva™ Catheter System Portfolio
Nexiva™ Catheter System is offered in four configurations. The dual port configurations allow for the simultaneous administration of two compatible solutions as close to the patient as possible. The single port configurations allow for the administration of one solution, as is common with most PIVCs.
Nexiva™ Catheter System is offered in configurations containing BD MaxZero™ Needle-free Connectors and BD Q-Syte™ Needle-free Connectors. MaxZero™ Needle-free Connectors are premium needle-free connectors and are offered on both dual port and single port configurations. BD Q-Syte™ Needle-free Connectors are also offered on dual port configuration.
- Paterson R, Larsen E, Cooke M, et al. Integrated versus non-integrated peripheral intravenous catheters: a cross-sectional survey of nurse experiences. Br J of Nurs. 2023, Vol 32.
- Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter-stabilization systems. J Infus Nurs. 2010;33(6):371-384.
- Infusion Nurses Society. Infusion therapy standards of practice. J Infus Nurs. 2024.
- Rickard CM, Larsen E, Walker Rachel M, et al. Integrated versus nonintegrated peripheral intravenous catheter in hospitalized adults (OPTIMUM): A randomized controlled trial. J Hosp Med. 2022;1-12.
- Rosenthal K. Get a hold on costs and safety with securement devices. Nurs Manage. 2005;36(5):52-53.
- Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs. 2015;38(3):189-203 doi: 10.1097/NAN.0000000000000100
- González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
- Carr PJ, Rippey JC, Cooke ML, et al. Development of a clinical prediction rule to improve peripheral intravenous cannulae first attempt success in the emergency department and reduce post insertion failure rates: the Vascular Access Decisions in the Emergency Room (VADER) study protocol. BMJ Open. 2016;6:e009196. doi.10.1136/bmjopen-2015-009196.
BD-3595 (07/24)
Clinical evidence
Nexiva™ Catheter System has been shown to deliver higher clinician satisfaction, improved clinician safety and a reduction in overall complication rates versus non-integrated, open systems.1–3
Nexiva™ Catheter System is backed by evidence in peer-reviewed journals from as early as 20094 to as recently as 2024.5
Nexiva™ Diffusics Closed IV Catheter System is also backed by peer-reviewed journals and infusion guidelines. Infusion Therapy Standards of Practice state to consider a fenestrated catheter for a contrast-based radiographic study and in coronary CTA.6 Nexiva Diffusics™ Catheter System is the only peripheral IV catheter with a diffusion catheter tip specifically designed for power injection.
Closed IV Catheter
| Comparative
| Data gathered | Year
| Study type | Journal | Authors |
| Nexiva™ | Various | Catheter failure, dislodgement, occlusion, phlebitis | 2024 | Systematic review/ meta analysis5 | Journal of Vascular Access | Gidaro A, Quici M, Giustivi D, et al. |
| Nexiva™ | BD Insyte Autoguard™ | Complications, dislodgement, dwell time, infiltration, occlusion, phlebitis | 2023 | RCT7 | European Journal of Oncology Nursing | Gerceker GO,
Yildirim BG, Onal A, et al. |
| Nexiva™ | B. Braun
BD Insyte | Catheter-associated bloodstream infection, clinician satisfaction, cost savings/utilization reduction, dislodgement/ leaking, dwell time, infiltration/extravasation, local infection, insertion success, occlusion, phlebitis | 2022 | RCT1 | Journal of Hospital Medicine | Rickard CM,
Larsen E, Walker RM, et al. |
| Nexiva™ | Smiths Jelco ProtectIV® Plus | Clinician satisfaction, dwell time, infection, infiltration, pain, phlebitis | 2019 | Prospective, quasi-experimental8 | Journal of Infusion Nursing | Penoyer D, Fowler S, Bennett M |
| Nexiva™ | None | Blood exposure, dislodgement, kinking, leaking, phlebitis, restarts | 2017 | Observational9 | Journal of Vascular Access | deRosenroll A |
| Nexiva™ | Medikit | Bending and kinking, blood exposure, cost savings/utilization reduction,dislodgement, dwell time, extravasation, insertion success, obstruction, phlebitis | 2014 | Quasi- randomized study10 | Journal of Vascular Access | Tamura N, Abe S, Hagimoto K, et al. |
| Nexiva™ | B. Braun Vasocan® Safety | Catheter-related infection, cost savings/utilization reduction, dwell time, infiltration/extravasation, insertion success, occlusion, pain, painful hematoma, phlebitis | 2014 | RCT2 | Journal of Hospital Infection | González López J, Arribi Vilela A, Fernández Del, Palacio E, et al. |
| Nexiva™ | B. Braun Introcan Safety® (non- winged) with StatLock | Clinician satisfaction, cost savings/utilization reduction, dislodgement, dwell time, infiltration, insertion success, leaking, phlebitis | 2010 | RCT3 | Journal of Infusion Nursing | Bausone-Gazda D, Lefaiver CA, Walters SA, et al. |
| Nexiva™ | None | Clinician satisfaction, clotting, dislodgement, infiltration, leaking, restarts, securement | 2009 | Pre-and post-use survey4 | Journal of the Association of Vascular Access | McNeil E, Hines N, Phariss R |
- Rickard CM, Larsen E, Walker Rachel M, et al. Integrated versus nonintegrated peripheral intravenous catheter in hospitalized adults (OPTIMUM): A randomized controlled trial. J Hosp Med. 2022;1-12.
- González López J, Arribi Vilela A, Fernández Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
- Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter-stabilization systems. J Infus Nurs. 2010;33(6):371-384.
- McNeil E, Hines N, Phariss R. A clinical trial of a new all-in-one peripheral-short catheter. JAVA. 2009;14(1):46-51.
- Gidaro A, Quici M, Giustivi D, et al. Integrated short peripheral intravenous cannulas and risk of catheter failure: a systematic meta-analysis. J Vasc Access. 2024.
- Infusion Nurses Society. Infusion therapy standards of practice. J Infus Nurs. 2024.
- Gerceker GO, Yildirim BG, Onal A, et al. The effect of the closed intravenous catheter system on first insertion success, indwelling time, and complications in pediatric hematology and oncology patients: a randomized controlled study. Eur J Oncol Nurs. 2023 Dec:67:102430. doi: 10.1016/j.ejon.2023.102430. Epub 2023 Oct 7.
- Penoyer D, Fowler S, Bennett M. Evaluation of the Use of Open Versus Closed Short Peripheral Catheters on Catheter Dwell Time. J Infus Nurs. 2019;42(6):276-282.
- deRosenroll A. Peripheral intravenous catheters: Improving outcomes through change in products, clinical practice and education. Vasc Access. 2017;11(1):7-12.
- Tamura N, Abe S, Hagimoto K, et al. Unfavorable peripheral intravenous catheter replacements can be reduced using an integrated closed intravenous catheter system. J Vasc Access. 2014;15(4):257-263.
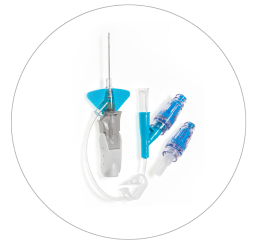
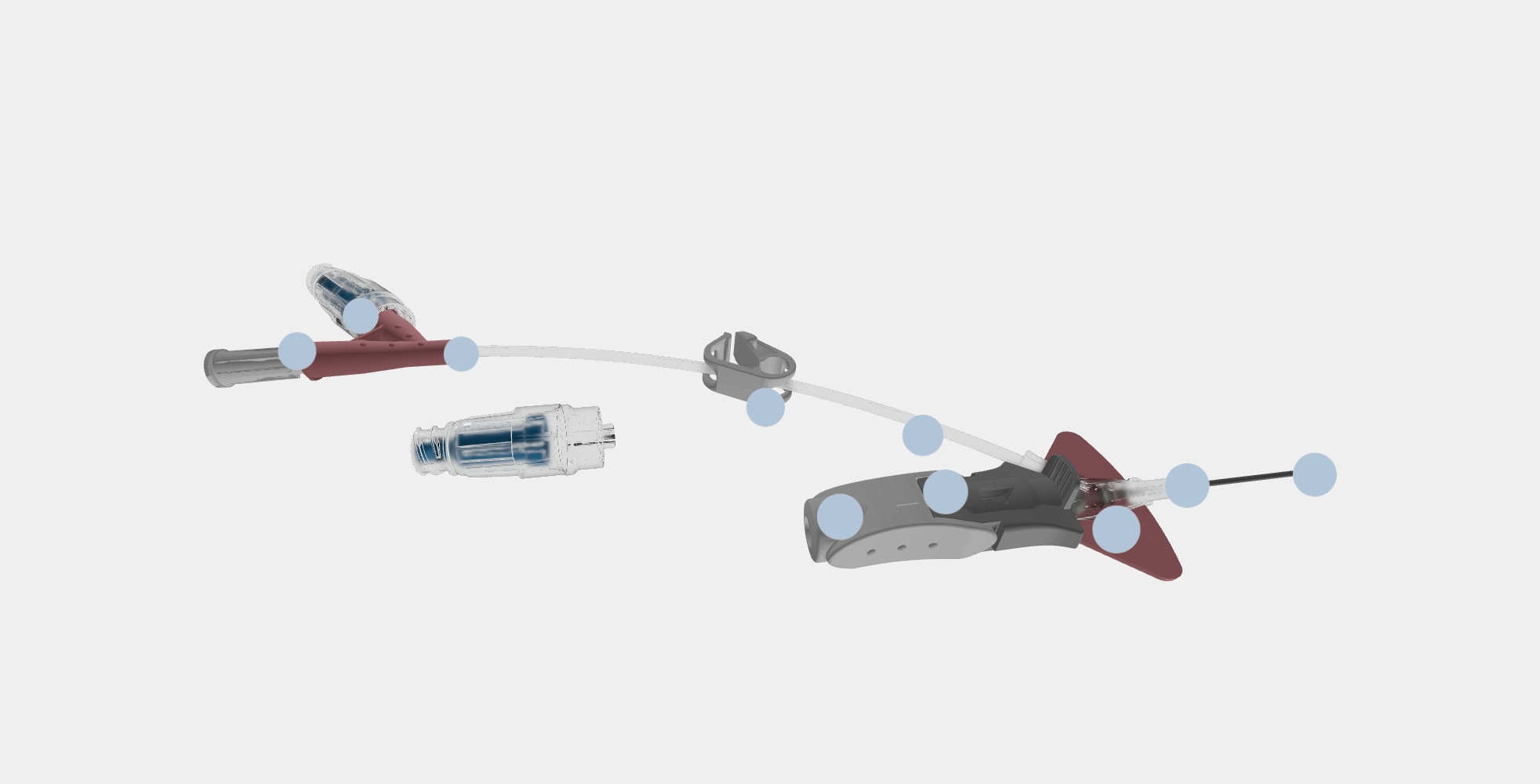
BD Nexiva™ Catheter System—Dual Port with BD MaxZero™ Needle-free Connectors
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
Vent plug
During insertion, the vent plug allows air to escape from the system enabling blood return to be seen in the catheter and extension tubing. The vent plug seals when blood reaches it, containing the blood within the system.
BD MaxZero™ Needle-free Connectors
Needle-free connectors close the system while enabling access for delivery of infusion therapy.
Needle-free connectors must be purchased separately from open/non-integrated PIVCs.
Dual port
The two ports allow for simultaneous administration of compatible solutions as close to the patient as possible. Non-integrated PIVCs require the second medication to be delivered through an injection port on the IV line.
Pinch clamp
The pinch clamp is used to control the flow of blood within the extension tubing and prevent blood leakage when replacing the vent plug or changing the needle-free connector. Each type of needle-free connector has a specified clamping sequence.
Pre-attached extension tube
The pre-attached extension tube, combined with the vent plug, creates a closed/integrated system that contains the blood as the catheter is inserted and serves as a secondary flashback chamber to support first attempt insertion success.
Ergonomic grip
The ergonomic grip is utilized during insertion and advancement of the catheter. After catheter advancement, pulling back on the white component releases the grey push tab component.
Passive safety mechanism
The passive safety mechanism fully encapsulates the needle tip as the needle is withdrawn and the gray push-tab component releases from the built-in stabilization platform. This helps reduce the risk of needlestick injury.
Stabilization platform
The built-in stabilization platform facilitates securement of the catheter to the patient and eliminates the need to purchase and add an external stabilization device, as is necessary with non-integrated PIVCs.
BD Vialon™ Catheter Material
The catheter resides within the vein and is made of a proprietary polyurethane named Vialon™ Catheter Material, which is differentiated from competitive polyurethane catheters and is utilized only in BD PIVCs.
BD Instaflash™ Needle Technology
Instaflash™ Needle Technology is the hole in the needle that allows blood to flow between the needle and the catheter so initial blood return is seen in the catheter. Blood continues to flow up the integrated extension set as secondary placement.
BD Nexiva™ Catheter System—Dual Port with BD MaxZero™ Needle-free Connectors
Catalog no. | Color | Gauge | Catheter
| Gravity flow rate
| Maximum power
|
| 383571 | Yellow | 24 | 0.75 | 18 | Not for use with power injectors |
| 383572 | Blue | 22 | 1.00 | 33 | 3.0 |
| 383573 | Blue | 22 | 1.75 | 30 | 3.0 |
| 383576 | Pink | 20 | 1.00 | 61 | 4.0—5.5 |
| 383577 | Pink | 20 | 1.25 | 58 | 4.0—5.5 |
| 383578 | Pink | 20 | 1.75 | 51 | 4.0—5.5 |
| 383579 | Green | 18 | 1.25 | 84 | 4.0—7.0 |
| 383580 | Green | 18 | 1.75 | 79 | 4.0—7.0 |
*22–18 G BD Nexiva™ Catheter System—Dual Port with BD MaxZero™ Needle-free Connectors attached are suitable for use with power injection. The catheter system has been tested at flow rates; however, due to variations in add-on devices, tubing, contrast media temperature and pressure limit settings these flow rates may not be achievable.
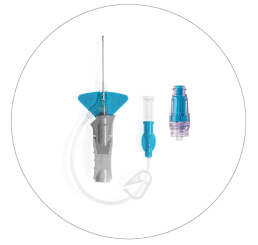
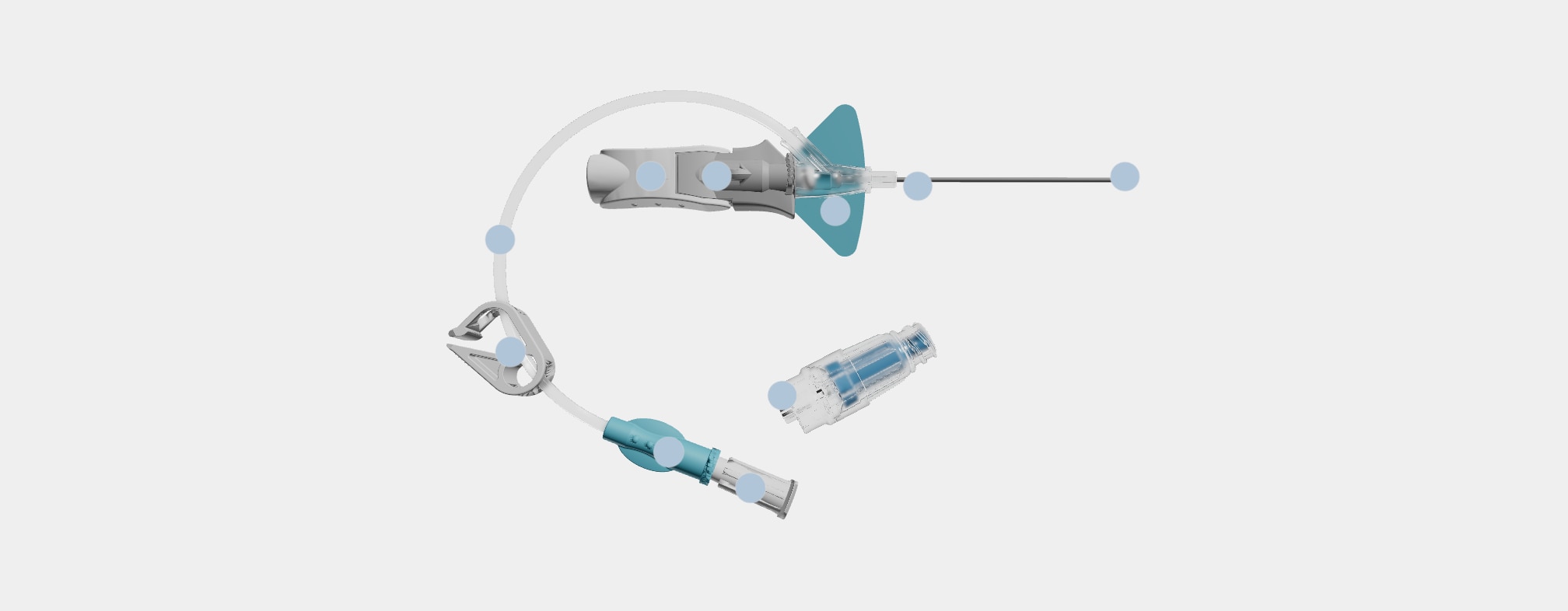
Nexiva™ Catheter System—Single Port with BD MaxZero™ Needle-free Connector
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
Vent plug
During insertion, the vent plug allows air to escape from the system enabling blood return to be seen in the catheter and extension tubing. The vent plug seals when blood reaches it, containing the blood within the system.
BD MaxZero™ Needle-free Connector
Needle-free connectors close the system while enabling access for delivery of infusion therapy.
Needle-free connectors must be purchased separately from open/non-integrated PIVCs.
Single port
The port is designed for the administration of a single solution after attaching a needle-free connector.
Pinch clamp
The pinch clamp is used to control the flow of blood within the extension tubing and prevent blood leakage when replacing the vent plug or changing the needle-free connector. Each type of needle-free connector has a specified clamping sequence.
Pre-attached extension tube
The pre-attached extension tube, combined with the vent plug, creates a closed/integrated system that contains the blood as the catheter is inserted and serves as a secondary flashback chamber to support first attempt insertion success.
Ergonomic grip
The ergonomic grip is utilized during insertion and advancement of the catheter. After catheter advancement, pulling back on the white component releases the grey push tab component.
Passive safety mechanism
The passive safety mechanism fully encapsulates the needle tip as the needle is withdrawn and the gray push-tab component releases from the built-in stabilization platform. This helps reduce the risk of needlestick injury.
Stabilization platform
The built-in stabilization platform facilitates securement of the catheter to the patient and eliminates the need to purchase and add an external stabilization device, as is necessary with non-integrated PIVCs.
BD Vialon™ Catheter Material
The catheter resides within the vein and is made of a proprietary polyurethane named Vialon™ Catheter Material, which is differentiated from competitive polyurethane catheters and is utilized only in BD PIVCs.
BD Instaflash™ Needle Technology
Instaflash™ Needle Technology is the hole in the needle that allows blood to flow between the needle and the catheter so initial blood return is seen in the catheter. Blood continues to flow up the integrated extension set as secondary placement.
Nexiva™ Catheter System—Single Port with BD MaxZero™ Needle-free Connector
Catalog no. | Color | Gauge | Catheter
| Gravity
| Max power
|
| 383550 | Yellow | 24 | 0.56 | 19 | Not for use with power injectors |
| 383551 | Yellow | 24 | 0.75 | 18 | Not for use with power injectors |
| 383552 | Blue | 22 | 1.00 | 33 | 3.0 |
| 383553 | Blue | 22 | 1.75 | 30 | 3.0 |
| 383556 | Pink | 20 | 1.00 | 61 | 4.0—5.5 |
| 383557 | Pink | 20 | 1.25 | 58 | 4.0—5.5 |
| 383558 | Pink | 20 | 1.75 | 51 | 4.0—5.5 |
| 383559 | Green | 18 | 1.25 | 84 | 4.0—7.0 |
| 393560 | Green | 18 | 1.75 | 79 | 4.0—7.0 |
*22–18 G BD Nexiva™ Catheter System — Single Port with BD MaxZero™ Needle-free Connectors attached are suitable for use with power injection. The catheter system has been tested at flow rates; however, due to variations in add-on devices, tubing, contrast media temperature and pressure limit settings these flow rates may not be achievable.
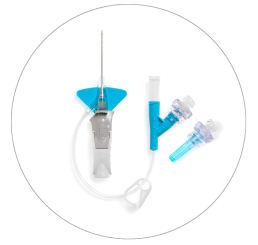
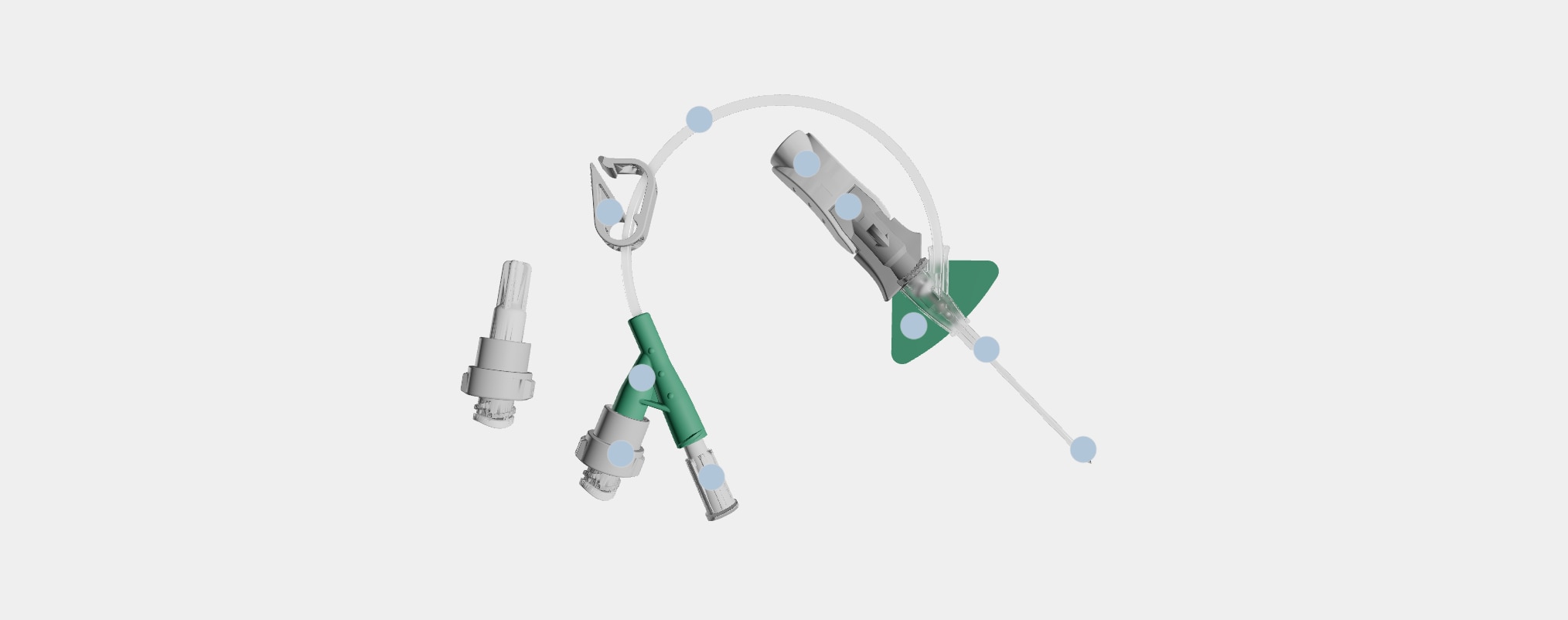
Nexiva™ Catheter System—Dual Port with Q-Syte™ Needle-free Connectors
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
Vent plug
During insertion, the vent plug allows air to escape from the system enabling blood return to be seen in the catheter and extension tubing. The vent plug seals when blood reaches it, containing the blood within the system.
BD Q-Syte™ Needle-free Connectors
Needle-free connectors close the system while enabling access for delivery of infusion therapy.
Needle-free connectors must be purchased separately from open/non-integrated PIVCs.
Dual port
The two ports allow for simultaneous administration of compatible solutions as close to the patient as possible. Non-integrated PIVCs require the second medication to be delivered through an injection port on the IV line.
Pinch clamp
The pinch clamp is used to control the flow of blood within the extension tubing and prevent blood leakage when replacing the vent plug or changing the needle-free connector. Each type of needle-free connector has a specified clamping sequence.
Pre-attached extension tube
The pre-attached extension tube, combined with the vent plug, creates a closed/integrated system that contains the blood as the catheter is inserted and serves as a secondary flashback chamber to support first attempt insertion success.
Ergonomic grip
The ergonomic grip is utilized during insertion and advancement of the catheter. After catheter advancement, pulling back on the white component releases the grey push tab component.
Passive safety mechanism
The passive safety mechanism fully encapsulates the needle tip as the needle is withdrawn and the gray push-tab component releases from the built-in stabilization platform. This helps reduce the risk of needlestick injury.
Stabilization platform
The built-in stabilization platform facilitates securement of the catheter to the patient and eliminates the need to purchase and add an external stabilization device, as is necessary with non-integrated PIVCs.
BD Vialon™ Catheter Material
The catheter resides within the vein and is made of a proprietary polyurethane named Vialon™ Catheter Material, which is differentiated from competitive polyurethane catheters and is utilized only in BD PIVCs.
BD Instaflash™ Needle Technology
Instaflash™ Needle Technology is the hole in the needle that allows blood to flow between the needle and the catheter so initial blood return is seen in the catheter. Blood continues to flow up the integrated extension set as secondary placement.
Nexiva™ Catheter System—Dual Port with Q-Syte™ Needle-free Connectors
Catalog no. | Color | Gauge | Catheter
| Gravity flow rate
| Maximum power
|
| 383530 | Yellow | 24 | 0.56 | 19 | Not for use with power injectors |
| 383531 | Yellow | 24 | 0.75 | 18 | Not for use with power injectors |
| 383532 | Blue | 22 | 1.00 | 33 | 3.0 |
| 383536 | Pink | 20 | 1.00 | 61 | 4.0—5.5 |
| 383537 | Pink | 20 | 1.25 | 58 | 4.0—5.5 |
| 383538 | Pink | 20 | 1.75 | 51 | 4.0—5.5 |
| 383539 | Green | 18 | 1.25 | 84 | 4.0—7.0 |
| 383540 | Green | 18 | 1.75 | 79 | 4.0—7.0 |
*BD Q-Syte™ Needle-free Connectors must be removed prior to power injection. The catheter system has been tested at flow rates; however, due to variations in add-on devices, tubing, contrast media temperature and pressure limit settings these flow rates may not be achievable.
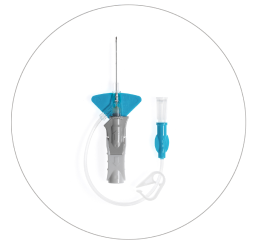
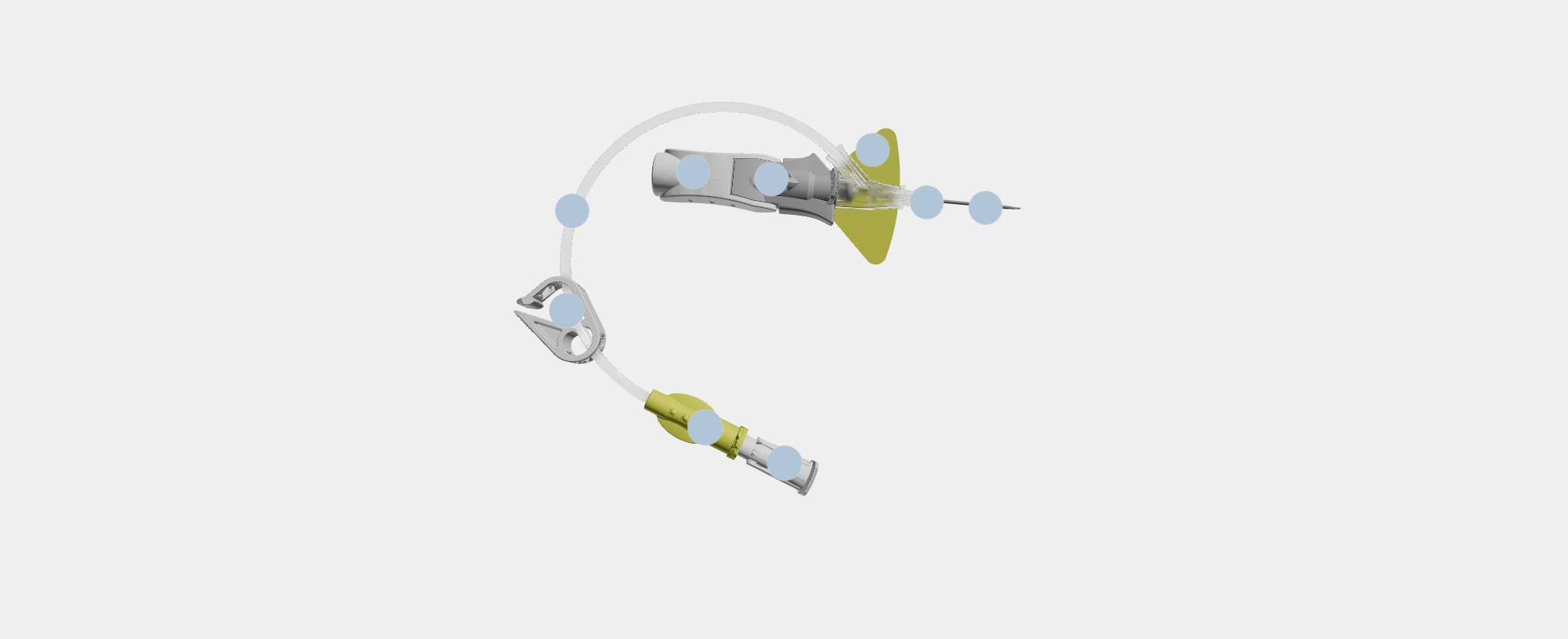
Nexiva™ Catheter System—Single Port
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
Vent plug
During insertion, the vent plug allows air to escape from the system enabling blood return to be seen in the catheter and extension tubing. The vent plug seals when blood reaches it, containing the blood within the system.
Single port
The port is designed for the administration of a single solution after attaching a needle-free connector.
Pinch clamp
The pinch clamp is used to control the flow of blood within the extension tubing and prevent blood leakage when replacing the vent plug or changing the needle-free connector. Each type of needle-free connector has a specified clamping sequence.
Pre-attached extension tube
The pre-attached extension tube, combined with the vent plug, creates a closed/integrated system that contains the blood as the catheter is inserted and serves as a secondary flashback chamber to support first attempt insertion success.
Ergonomic grip
The ergonomic grip is utilized during insertion and advancement of the catheter. After catheter advancement, pulling back on the white component releases the grey push tab component.
Passive safety mechanism
The passive safety mechanism fully encapsulates the needle tip as the needle is withdrawn and the gray push-tab component releases from the built-in stabilization platform. This helps reduce the risk of needlestick injury.
Stabilization platform
The built-in stabilization platform facilitates securement of the catheter to the patient and eliminates the need to purchase and add an external stabilization device, as is necessary with non-integrated PIVCs.
BD Vialon™ Catheter Material
The catheter resides within the vein and is made of a proprietary polyurethane named Vialon™ Catheter Material, which is differentiated from competitive polyurethane catheters and is utilized only in BD PIVCs.
BD Instaflash™ Needle Technology
Instaflash™ Needle Technology is the hole in the needle that allows blood to flow between the needle and the catheter so initial blood return is seen in the catheter. Blood continues to flow up the integrated extension set as confirmation of placement.
Nexiva™ Catheter System—Single Port
Catalog no. | Color | Gauge | Catheter
| Gravity flow rate
| Maximum power
|
| 383510 | Yellow | 24 | 0.56 | 19 | Not for use with power injectors |
| 383511 | Yellow | 24 | 0.75 | 18 | Not for use with power injectors |
| 383512 | Blue | 22 | 1.00 | 33 | 3.0 |
| 383513 | Blue | 22 | 1.75 | 30 | 3.0 |
| 383516 | Pink | 20 | 1.00 | 61 | 4.0—5.5 |
| 383517 | Pink | 20 | 1.25 | 58 | 4.0—5.5 |
| 383518 | Pink | 20 | 1.75 | 51 | 4.0—5.5 |
| 383519 | Green | 18 | 1.25 | 84 | 4.0—7.0 |
| 383520 | Green | 18 | 1.75 | 79 | 4.0—7.0 |
*The catheter system has been tested at flow rates; however, due to variations in add-on devices, tubing, contrast media temperature and pressure limit settings these flow rates may not be achievable.
Find our training resources to help improve your clinical practices as part of our goal of advancing the world of health.
Find our training resources to help improve your clinical practices as part of our goal of advancing the world of health.