BD, the BD Logo, Advancing the World of Health, AllPoints, C.R.Bard, ChronoFlex, ClearVUE, Groshong, MiniLoc, M.R.I., PortReady, PowerFlow, PowerPort, PowerLoc, SafeStep, SlimPort, Rosenblatt and the radiopaque identifier are trademarks of Becton, Dickinson and Company or its affiliates. All other trademarks are the property of their respective owners. © 2024 BD. All rights reserved.
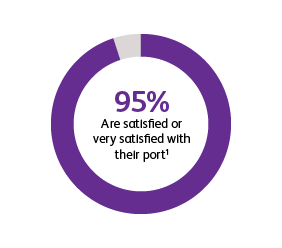
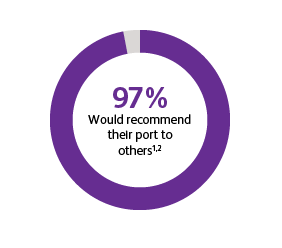
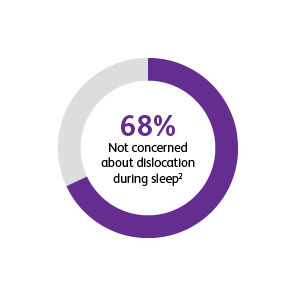
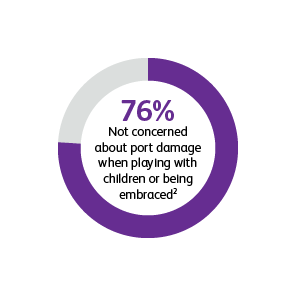
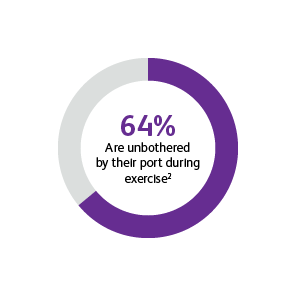
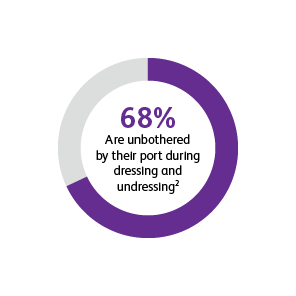
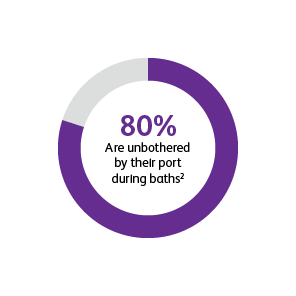
* Two surveys were conducted to compare patients’ satisfaction and impact on daily life after port implantation. Data is from patients who were surveyed 90-days after their port implantation. Goltz et al., 2013 was a prospective study that surveyed 25 consecutive patients with chest-implanted ports who had an underlying malignancy with upcoming chemotherapy.1,2 All chest-implanted ports were PowerPort™ isp Implantable Ports, Bard Access Systems, Salt Lake City, Utah, USA. The questionnaire was developed by two of the authors and covered four topics: (a) information about and perception of the implantation procedure; (b) comfort of the port, influence on daily activities, cosmetic aspects, and existence of certain fears regarding device function; (c) history of device-related complications; and (d) overall satisfaction with the port device. Kunz-Virk et al., 2019 conducted a telephone interview to survey 89 patient’s (90% requiring the port for chemotherapy) subjective rating of the port.2 All ports were PowerPort™ isp Implantable Ports MR 6.0 French (Fr), Attachable Polyurethane Catheter, Bard Access Systems, Salt Lake City, Utah, USA. Patients were surveyed 90-days post implantation using a standardized questionnaire.1,2
1 Kunz-Virk J, Krüger K. Power-injectable totally implantable venous access devices - analysis of success and complication rates of ultrasound-guided implantation and a patient satisfaction survey. Vasa. 2019;48(6):524-530. doi:10.1012 /0301-1526/a000802
2 Goltz JP, Petritsch B, Kirchner J, Hahn D, Kickuth R. Percutaneous image-guided implantation of totally implantable venous access ports in the forearm or the chest? A patients’ point of view. Supportive Care in Cancer. 2013;21:505-510.
BD-138406