BD offers a cutting-edge portfolio of ID/AST platforms made up of the BD Phoenix™ M50, BD Phoenix™ AP, BD Bruker MALDI Biotyper®, Accelerate Pheno® System and Accelerate Arc™ System that can inform effective antimicrobial optimization through robust testing capabilities and a uniquely phenotypic-focused approach. By examining the observable traits and characteristics of microorganisms, this method provides a direct and practical understanding of how they respond to different antimicrobial agents. This phenotypic approach is aligned with the goal of providing easily interpretable, actionable results that reflect the real-time and comprehensive phenotypic activity of each microorganism, which is crucial as resistance mechanisms become increasingly complex.24
The BD® Automated Identification and Antimicrobial Susceptibility Testing Solution supports your bloodstream infection diagnostic pathway by standardizing workflows and providing timely and accurate detection of known and emerging antimicrobial resistance that supports your antimicrobial stewardship efforts.3-10
While this robust instrument platform supports the bloodstream infection diagnostic pathway, it also provides results for most aerobic and facultative anaerobic gram-positive and gram-negative bacteria of human origin from subcultures from a variety of specimen types encountered in the laboratory (urine, wound, respiratory, stool), ensuring comprehensive and efficient testing for various infections.3
The integrated BD ID/AST solution addresses your identification and susceptibility testing needs with speed, accuracy and efficiency, by offering both extensive pathogen testing solutions and fast identification and antimicrobial susceptibility testing options for critically ill patients.1-3

BD Phoenix™ M50 Automated Microbiology System
With an extensive panel well count and antimicrobial offering, true doubling dilutions, and extended dilutions for lower breakpoints, the BD Phoenix™ M50 System marries performance with ease of use to provide the timely and accurate pathogen identification and susceptibility results that clinicians need to help guide their therapy and patient management decisions.3-8
In addition to accurately confirming known resistance, the BD Phoenix™ M50 System has demonstrated performance in detecting newly emerging resistance to antimicrobials that historically have been susceptible.3,4

BD Phoenix™ AP Instrument
The BD Phoenix™ AP sample preparation instrument is your automated solution for inoculum preparation.
The BD Phoenix™ AP Instrument automates human processes, decreasing technologist time and labor to prepare the panels, standardizing inoculum preparation and incorporating automated nephelometry.9,10

Robust identification and comprehensive coverage
The BD™ Bruker MALDI Biotyper® CA System offers high accuracy of organism identification, with high-confidence ID results having comparable accuracy to 16S rRNA gene sequencing.16
We have an intentional focus on library expansion which currently offers US-IVD identification for over 480 different FDA-cleared species including gram-negative bacteria, gram-positive bacteria, and yeasts.
The proprietary lifetime** Smartbeam™ laser and high-performance vacuum of the BD™ Bruker MALDI Biotyper® CA System help generate fast target exchange times.

Expedite antibiotic therapy decisions with fast actionable phenotypic susceptibility results
Acutely ill patients with septic shock cannot wait for optimal antimicrobial treatment and clinicians need timely actionable results to inform treatment decisions and improve patient outcomes.6,23
With the speed and simplicity of the Accelerate Pheno® system, microorganism identification and actionable phenotypic susceptibility results based on minimum inhibitory concentrations (MIC) are available in approximately 7 hours directly from positive blood culture samples, supporting faster initiation of optimal therapy and expanding the value of antimicrobial stewardship.24-30

MALDI prep, simplified
The Accelerate Arc™ System provides an automated rapid clean-up of positive blood cultures from a BD BACTEC™ bottle for direct ID by BD MALDI Biotyper® in ~90 minutes.19
Just 2-3 minutes of hands-on time is all it takes for an Accelerate Arc module and BC kit. Simple enough to run on all shifts by any technician in the lab.19
Your total cost to rapidly identify organisms from positive blood cultures could be at least 50% less than what you are paying for a rapid molecular ID solution.20-23

BD Phoenix™ Panels
BD Phoenix™ panels require no refrigeration, are leak resistant and are available in a variety of formats including identification-only, susceptibility-only, and combination identification and susceptibility panels.
BD Phoenix™ panels provide accurate, rapid identification and antimicrobial susceptibility testing results for most clinically significant aerobic and facultative anaerobic Gram-negative and Gram-positive bacteria, as well as identification of yeast and yeast-like organisms.
Choose the BD Automated Identification and Antimicrobial Susceptibility Testing Solutions as part of the integrated, end-to-end BD Bloodstream Infection Solution that seamlessly connects specimen collection, blood culture and antimicrobial susceptibility testing.
Related Products
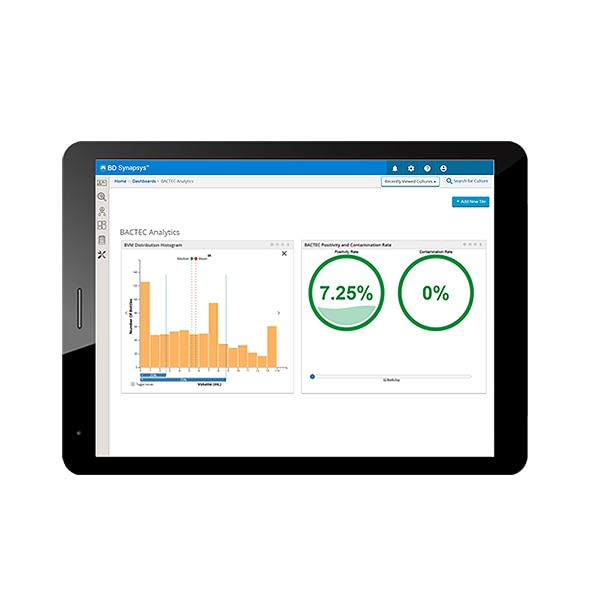
Meaningful and actionable insights accessible anytime, anywhere with BD Informatics
- Accelerate PhenoTest BC kit Instructions for Use (LBL000172).
- BD™ Bruker MALDI Biotyper CA System User Manual (8603291).
- BD Phoenix™ M50 Automated Microbiology System User's Manual (500008930).
- Carey RB et al. Failure of Automated Systems to Detect Vancomycin-Resistant Staphylococcus aureus. Abstract presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Washington, DC, 2004.
- Tenover FC et al. Emerg Infect Dis. 2006;12(8):1209–13.
- Kulah C et al. BMC Infect Dis. 2009;9:30.
- Mittman SA et al. J Clin Microbiol. 2009;47(11):3557–61.
- Woodford N et al. J Clin Microbiol. 2010;48(8):2999–3002.
- Junkins A et al. J. Clin. Microbiol. 2010;48(5):1929–31.
- Jungkind D et al. Workflow evaluation of the BD Phoenix™ AP Compared to the MicroScan Walkaway overnight system for identification and susceptibility testing of bacteria. Poster presented at the 110th General Meeting of the American Society of Microbiology. May 2010. Study was supported by grants from BD Diagnostic Systems.
- Walsh TL et al. Infection. 2021;49(3):511–9.
- Sheth S et al. Antimicrob Agents Chemother. 2020;64(9):e00578–20
- Bhalodi AA et al. Clin Infect Dis. 2022;75(2):269–77.
- Saffert RT et al. J Clin Microbiol. 2011;49(3):887–92.
- Knabl L et al. Lett Appl Microbiol. 2021;73(1):2–8.
- Watanabe N et al. J Infect Chemother. 2022;28(4):563–8.
- BD EpiCenter™ Microbiology Data Management System User's Training Manual (SHQ-TM-020).
- BD Synapsys™ Microbiology Informatics Solution User's Manual (L011216).
- Accelerate Arc™ BC Kit Instructions for Use, (LBL000774 Rev. 1)
- Pardo et al. ASM 2014
- Doern et al. Clinical Microbiology Review 2019
- https://www.gsaadvantage.gov/ref_text/V797D30085/0XCQE4.3T33AP_V797D-30085_BIOFIREVATSCSJULY.PDF
- Accelerate Arc™ Module, MKT000289_A(1672) axdx.com