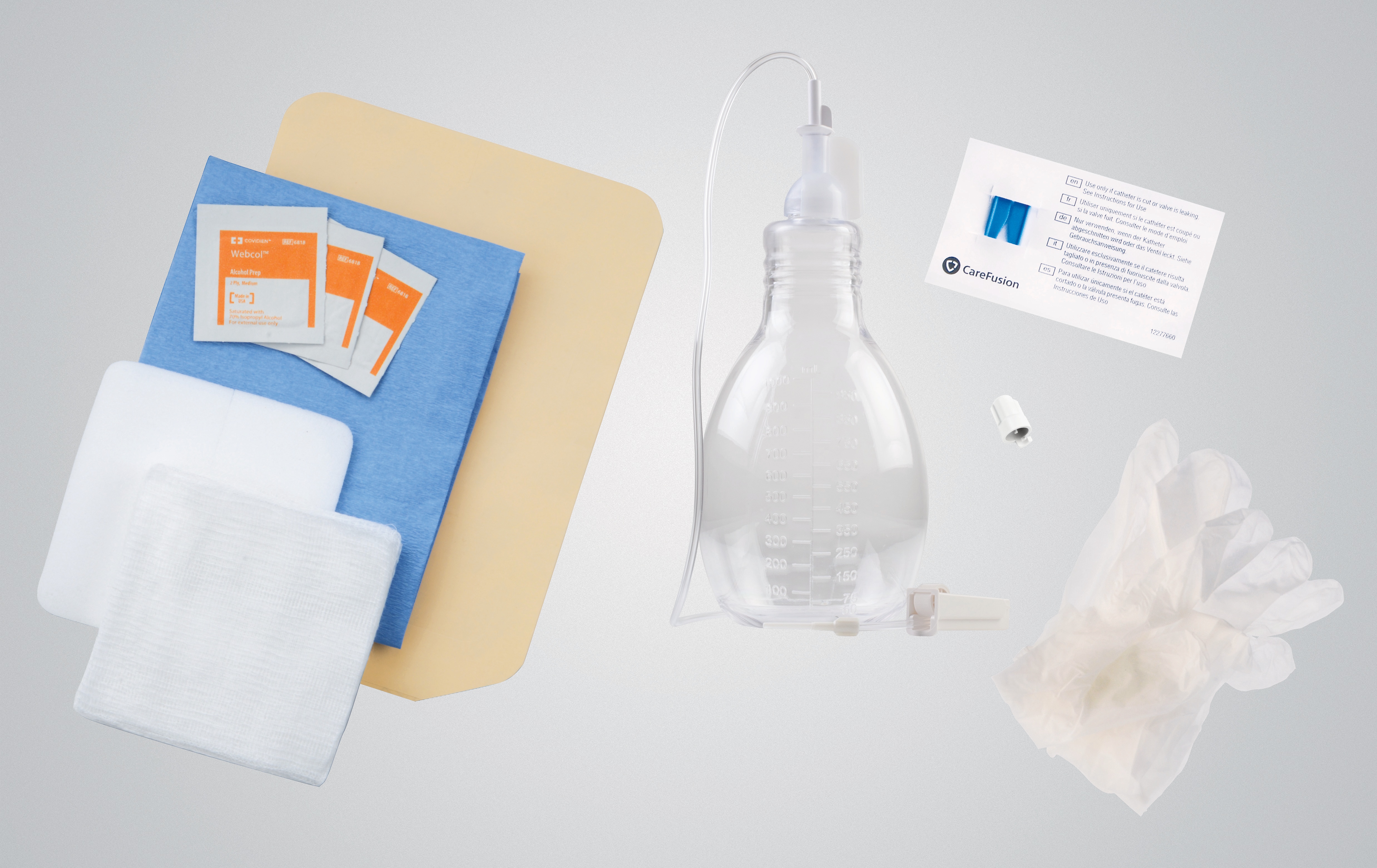
PeritX™ Peritoneal Catheter System
Indication for Use
The PeritX™ Peritoneal Catheter System is indicated for intermittent, long term drainage of symptomatic, recurrent, malignant or non- malignant ascites that does not respond to medical management of the underlying disease and for the palliation of symptoms related to recurrent ascites. The use of the PeritX™ Peritoneal Catheter for non-malignant ascites is limited to patients who are intolerant or resistant to maximum medical therapy, refractory to large volume paracentesis (LVP) and are not candidates for a trans-jugular intrahepatic portosystemic shunt or LVP. The PeritX™ Peritoneal Catheter is indicated for adults only.
The Lockable Drainage Line is used to drain fluid using standard wall suction, water seal drainage system, vacuum bottle, or other appropriate method.
Contraindications
Use of the PeritX™ Peritoneal Catheter System is contraindicated in the following situations:
1. When the peritoneal cavity is multi-loculated, and the drainage of a single loculation would not be expected to provide relief of dyspnea or other symptoms.
2. When there is a coagulopathy.
3. When the peritoneal cavity is infected.
4. When the patient is known or suspected to be allergic to materials contained in the device.
The valved peel-away introducer and catheter insertion stylet are not designed for use in the arterial system or as a hemostatic device.
Warnings
Do not put anything except the access tip of the Lockable Drainage Line, Catheter Access Kit, or Vacuum Bottles into the PeritX™ Catheter valve since any other device could damage the valve. A damaged valve may allow air into the body or let fluid leak out through the valve when not draining.
A diagnostic paracentesis should be performed if the patient shows any signs or symptoms of possible spontaneous bacterial peritonitis (SBP ) such as fever or abdominal pain. The patient should be evaluated and treated per institutional guidelines.
Cautions
For single use only. Re-use may result in a nonfunctional product or contribute to cross contamination.
Sterile technique should be used when placing and draining the catheter.
Care must be taken not to bend or kink the sheath.
Damage to the sheath may prevent passage of the catheter.
The fenestrations must be entirely within the peritoneal space to avoid leakage into the tunnel tract. Take patient size, tunnel length, and the catheter length into account when placing the catheter.
The valved peel-away introducer is designed to reduce fluid loss and the risk of air intake, but is not a hemostasis valve. It is not intended to create a two-way seal, nor for arterial use. It will substantially reduce the race of fluid flow, but some fluid loss through the valved peel-away introducer may occur.
Indications for Use:
The PeritX™ Drainage Kits are indicated for use only with the PeritX™ Peritoneal Catheter for intermittent drainage.
Warnings:
Examine the product to ensure it has not been damaged during shipment and that its size, shape, and condition are suitable for the procedure for which it is to be used. Do not use if product damage or contamination is evident.
This device has been designed for single use only. Reusing this medical device bears the risk of cross-patient contamination as medical devices – particularly those with long and small lumina, joints, and/or crevices between components – are difficult or impossible to clean once body fluids or tissues with potential pyrogenic or microbial contamination have had contact with the medical device for an indeterminable period of time. The residue of biological material can promote the contamination of the device with pyrogens or microorganisms which may lead to infectious complications. Additionally, re-use and/or repackaging may compromise the structural integrity and/or material and design characteristics of the device, which may lead to device failure, and/or lead to patient injury.
Do not resterilize. After resterilization, the sterility of the product is not guaranteed because of an indeterminable degree of potential pyrogenic or microbial contamination which may lead to infectious complications. Cleaning, reprocessing and/or resterilization of the present medical device increases the probability that the device will malfunction due to potential adverse effects on components that are influenced by thermal and/or mechanical changes.
After use, this product may be a potential biohazard. Dispose of the drained liquid and the used drainage bag per applicable local regulations. Handle and dispose of in accordance with accepted medical practice and local, state and federal laws and regulations. This product should be disposed of appropriately, not resterilized. Do not reuse.
This device contains polyvinyl chloride (PVC), which is known to potentially use plasticizers such as, Di(2-ethylehexyl) phthalate (DEHP). DEHP has been shown to produce a range of adverse effects in experimental animals, notably liver toxicity and testicular atrophy. Although the toxic and carcinogenic effects of DEHP have been well established in experimental animals, the ability of this compound to produce adverse effects in humans is controversial. BD has not assessed any related adverse effects in relation to the exposure to DEHP when this device is used by pregnant or breastfeeding women. It is the responsibility of the physician to assess risks associated with the use of a device containing DEHP. 1
WARNING: This product can expose you to chemicals including Di(2-ethylhexyl) phthalate (DEHP), which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Do not use scissors or other sharp objects near the PeritX™ Peritoneal Catheter.
Keep the valve on your catheter and the access tip on the drainage line clean. Keep them away from other objects to help avoid contamination.
Do not put anything except the access tip into the catheter valve since any other device could damage the valve. A damaged valve may allow air into your body or let fluid leak out through the valve when you are not draining.
Do not drain more than 2000 ml of fluid from your abdomen at any one time.
It is normal to feel some discomfort or pain when draining fluid. If discomfort or pain is experienced when draining, push the flow control slider on the handheld unit away from the bag to slow or stop the flow of fluid for a few minutes. If you don’t feel better after doing this, or the pain is severe, consult your doctor or nurse. Pain may be an indication of infection.
Keep your gloves clean. If they become contaminated, wash your hands and put on a new pair of gloves prior to connecting a new bag.
The PeritX™ Drainage Bag has not been tested with any other peritoneal catheters except the PeritX™ Peritoneal Catheter for intermittent drainage. If the catheter is cut or the valve is leaking, follow these steps:
- Pinch the catheter closed between your fingers.
- Slip the blue emergency slide clamp (Refer to Figure 2) over the catheter and push the catheter completely into the small end of the clamp. This will close the catheter.
- Notify your doctor immediately.
Precautions
The alcohol pads are flammable. Do not expose the pads to an open flame.
The Procedure Pack and Drainage Bag are not made with natural rubber latex.
Pain, redness (erythema), warmth to touch, swelling (edema), fever, or fluid around the catheter site may indicate your catheter is infected. Some discomfort and redness after insertion is expected but should not persist or worsen. If you see signs of infection, finish the drainage procedure and consult your doctor or nurse.
Make sure that the valve and the access tip are securely connected when draining. If they are accidentally separated, they may become contaminated. If this occurs, clean the valve with an alcohol pad and use a new drainage bag to avoid potential contamination.
Ensure that the drainage line is not tugged or pulled.
Loops or kinks in the tubing may cause fluid flow to stop early. If this occurs, remove the kink or loop and resume draining.
If fluid spills, use soap and water to clean skin and use appropriate cleaning agent for all other surfaces.
Contact your physician if:
- You believe your catheter is infected. Pain, redness (erythema), warmth to touch, swelling (edema), fever, or fluid around the catheter site may indicate your catheter is infected. Some discomfort and redness after insertion is expected but should not persist or worsen.
- Shortness of breath is not relieved after draining 2000 ml of fluid from the abdomen at one time.
- You continue to experience symptoms, but little or no fluid drains from the catheter.
- The appearance (color, thickness, etc.) or amount drained changes significantly between drainages.
Potential Complications
Potential complications of draining fluid from the abdomen include, but may not be limited to, hypotension, circulatory collapse, electrolyte imbalance, protein depletion, ascites leakage, peritonitis, and wound infection.
Note: Users and/or patients within the European Union should report any serious incident that has occurred in relation to the device to the manufacturer and the competent authority of the Member State in which the user and/or patient is established. Users outside of the European Union should report any serious incident that has occurred in relation to the device to the manufacturer and the regulatory authority of the country in which the user and/or patient is established.
PeritX™ Drainage Bag
Indication for Use
The PeritX™ Drainage Kits are indicated for use only with the PeritX™ Peritoneal Catheter for intermittent drainage.
Warnings
Examine the product to ensure it has not been damaged during shipment and that its size, shape, and condition are suitable for the procedure for which it is to be used. Do not use if product damage or contamination is evident.
This device has been designed for single use only. Reusing this medical device bears the risk of cross-patient contamination as medical devices – particularly those with long and small lumina, joints, and/or crevices between components – are difficult or impossible to clean once body fluids or tissues with potential pyrogenic or microbial contamination have had contact with the medical device for an indeterminable period of time. The residue of biological material can promote the contamination of the device with pyrogens or microorganisms which may lead to infectious complications. Additionally, re-use and/or repackaging may compromise the structural integrity and/or material and design characteristics of the device, which may lead to device failure, and/or lead to patient injury.
Do not resterilize. After resterilization, the sterility of the product is not guaranteed because of an indeterminable degree of potential pyrogenic or microbial contamination which may lead to infectious complications. Cleaning, reprocessing and/or resterilization of the present medical device increases the probability that the device will malfunction due to potential adverse effects on components that are influenced by thermal and/or mechanical changes.
After use, this product may be a potential biohazard. Dispose of the drained liquid and the used drainage bag per applicable local regulations. Handle and dispose of in accordance with accepted medical practice and local, state and federal laws and regulations. This product should be disposed of appropriately, not resterilized. Do not reuse.
This device contains polyvinyl chloride (PVC), which is known to potentially use plasticizers such as, Di(2-ethylehexyl) phthalate (DEHP). DEHP has been shown to produce a range of adverse effects in experimental animals, notably liver toxicity and testicular atrophy. Although the toxic and carcinogenic effects of DEHP have been well established in experimental animals, the ability of this compound to produce adverse effects in humans is controversial. BD has not assessed any related adverse effects in relation to the
exposure to DEHP when this device is used by pregnant or breastfeeding women. It is the responsibility of the physician to assess risks associated with the use of a device containing DEHP.
WARNING: This product can expose you to chemicals including Di(2-ethylhexyl) phthalate (DEHP), which is known to the State of California to cause cancer and birth defects or other reproductive harm. For more information go to www.P65Warnings.ca.gov.
Do not use scissors or other sharp objects near the PeritX™ Peritoneal Catheter.
Keep the valve on your catheter and the access tip on the drainage line clean. Keep them away from other objects to help avoid contamination.
Do not put anything except the access tip into the catheter valve since any other device could damage the valve. A damaged valve may allow air into your body or let fluid leak out through the valve when you are not draining.
Do not drain more than 2000 ml of fluid from your abdomen at any one time.
It is normal to feel some discomfort or pain when draining fluid. If discomfort or pain is experienced when draining, push the flow control slider on the handheld unit away from the bag to slow or stop the flow of fluid for a few minutes. If you don’t feel better after doing this, or the pain is severe, consult your doctor or nurse. Pain may be an indication of infection.
Keep your gloves clean. If they become contaminated, wash your hands and put on a new pair of gloves prior to connecting a new bag.
The PeritX™ Drainage Bag has not been tested with any other peritoneal catheters except the PeritX™ Peritoneal Catheter for intermittent drainage. If the catheter is cut or the valve is leaking, follow these steps:
1. Pinch the catheter closed between your fingers.
2. Slip the blue emergency slide clamp (Refer to Figure 2) over the catheter and push the catheter completely into the small end of the clamp. This will close the catheter.
3. Notify your doctor immediately.
Precautions
The alcohol pads are flammable. Do not expose the pads to an open flame.
The Procedure Pack and Drainage Bag are not made with natural rubber latex.
Pain, redness (erythema), warmth to touch, swelling (edema), fever, or fluid around the catheter site may indicate your catheter is infected. Some discomfort and redness after
insertion is expected but should not persist or worsen. If you see signs of infection, finish the drainage procedure and consult your doctor or nurse.
Make sure that the valve and the access tip are securely connected when draining. If they are accidentally separated, they may become contaminated. If this occurs, clean the valve with an alcohol pad and use a new drainage bag to avoid potential contamination.
Ensure that the drainage line is not tugged or pulled.
Loops or kinks in the tubing may cause fluid flow to stop early. If this occurs, remove the kink or loop and resume draining.
If fluid spills, use soap and water to clean skin and use appropriate cleaning agent for all other surfaces. Contact your physician if:
• You believe your catheter is infected. Pain, redness (erythema), warmth to touch, swelling (edema), fever, or fluid around the catheter site may indicate your catheter is infected. Some discomfort and redness after insertion is expected but should not persist or worsen.
• Shortness of breath is not relieved after draining 2000 ml of fluid from the abdomen at one time.
• You continue to experience symptoms, but little or no fluid drains from the catheter.
• The appearance (color, thickness, etc.) or amount drained changes significantly between drainages.
Potential Complications
Potential complications of draining fluid from the abdomen include, but may not be limited to, hypotension, circulatory collapse, electrolyte imbalance, protein depletion, ascites leakage, peritonitis, and wound infection.
Note: Users and/or patients within the European Union should report any serious incident that has occurred in relation to the device to the manufacturer and the competent authority of the Member State in which the user and/or patient is established. Users outside of the European Union should report any serious incident that has occurred in relation to the device to the manufacturer and the regulatory authority of the country in which the user and/or patient is established.
Please consult package insert for more detailed safety information and instructions for use.
BD-146833