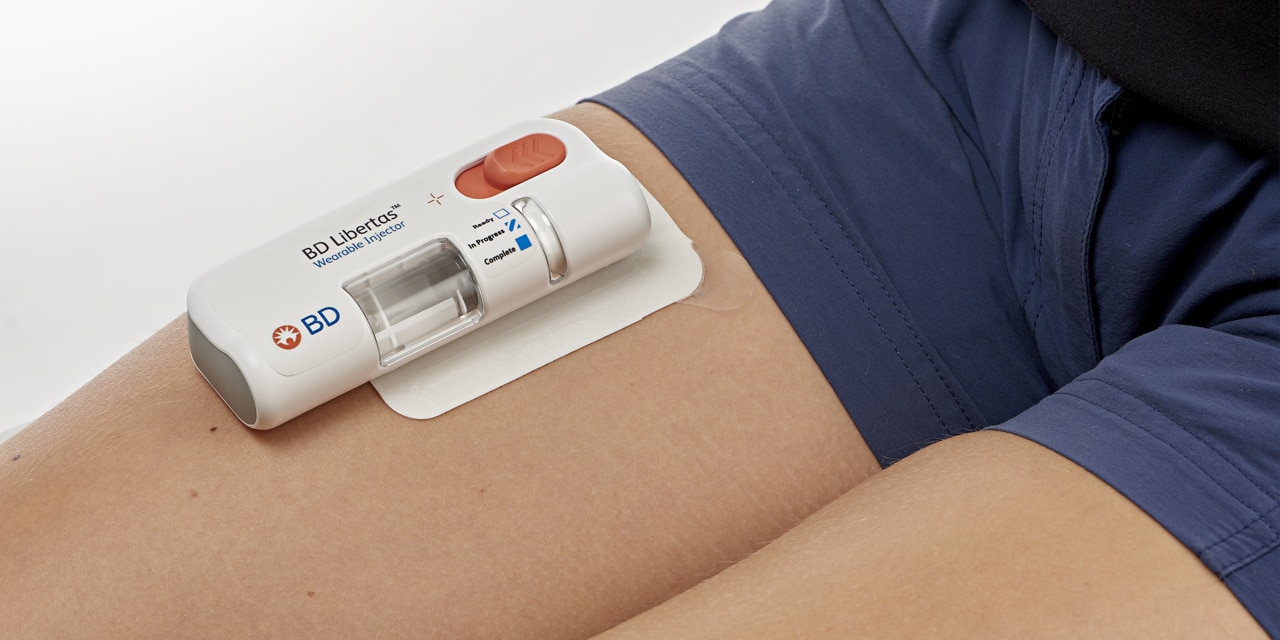
BD Libertas™ Wearable Injector
Designed to deliver large volume and/or high viscosity fixed dose subcutaneous injection.1,*


- Overview
- Expertise
- Availability & Support
- Products & Accessories
- Resources
The BD Libertas™ Wearable Injector is designed as a pre-fillable drug delivery system for combination products.1
The BD Libertas™ Wearable Injector is an innovative injection system designed to deliver complex biologics with viscosities up to 50 cP in 2-5 mL and 5-10 mL configurations, at home or in clinical settings.1
BD Libertas™ Wearable Injector features a mechanical spring-based power source, without battery or heavy metals disposal concerns.1, 2
BD reinforced its commitment to innovation by completing a 52-subject human clinical trial for its award-winning¥ 2-5 mL BD Libertas™ Wearable Injector.3
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
BD Libertas™ Wearable Injector - Introductory overview video
BD Libertas™ Wearable Injector Features
- Single use, fixed dose, prefillable design, with pre-attached adhesive pad
- Dose delivery options: 2-5 mL or 5-10 mL
- Mechanical spring-based power module
- BD glass primary container and needle technology
- Transparent viewing window for drug and drug delivery visibility
- Push button activation
- Color-coded status indicator
BD Libertas™ Wearable Injector Benefits
- Ready-to-use, simple, design requiring no patient filling or assembly1
- Enables hands-free drug delivery1
- Designed to conceal the needle before and after injection1
- Automated needle insertion and passive needle retraction1
- Designed with multiple indications of injection status (audible, tactile, visual) throughout the injection process1
- No battery or heavy metals disposal concerns1,2
- Delivered as five component modules in sterile packaging for factory fill/finish and assembly1
- Designed for pharma fill/finish utilizing standardized syringe tubs and commercial prefillable syringe filling equipment and processes1
- Primary containers available in standard nest and tub configurations2
- Stoppers available in TSCF packaging2
- No software or electronics supplier management and revision controls required2
BD Libertas™ Wearable Injector is a product in development; some statements are forward looking and are subject to a variety of risks and uncertainties. BD Libertas™ Wearable Injector is a device component intended for drug-device combination products and not subject to FDA 510(k) clearance or separate EU CE mark certification.
* large volume: 2-5 mL or 5-10 mL. - high viscosity: up to 50 cP.
¥ BD Libertas™ Wearable Injector and DCA Design International have won two design awards: the Good Design® Award and the iF Design Excellence Award.
- Design Input Specification for BD Libertas™ Platform [Internal report]. Franklin Lakes, USA: Becton, Dickinson and Company; 2014. Revision: 05
- Design Output Specification for BD Libertas™ Platform [Internal report]. Franklin Lakes, USA: Becton, Dickinson and Company; 2017. Revision: 04
- Woodley, W. D. et al. Clinical Evaluation of an Investigational 5ml Wearable Injector in Healthy Human Subjects. Clin Transl Sci. 2021 May; 14(3):859-869. doi: 10.1111/cts.12946.
BD has conducted over 50 BD pre-clinical4 and clinical5 studies to inform the design and development of the BD Libertas™ Wearable Injector, demonstrate feasibility of 2-10 mL biologic injections into subcutaneous tissue and characterize tissue response to large volume injections in human and animal subjects.3
BD conducted a 52-subject human clinical trial with the BD Libertas™ Wearable Injector that evaluated the performance of the 2-5 mL device, including tissue effects, tolerability (pain) and patient acceptance.3 100% of study subjects likely to use if prescribed.3
Results of this human clinical trial are published in Clinical and Translational Science.3
Additional BD research into large volume subcutaneous injections may be found in another published clinical study in the peer reviewed journal Clinical and Translational Science. This additional translational feasibility study examined an expanded range of volumes and viscosities (up to 10ml & 20cP) using delivery from a surrogate syringe pump and without co-delivery of a permeation enhancer in 32 healthy adult subjects.
BD Libertas™ Wearable Injector is a product in development; some statements are forward looking and are subject to a variety of risks and uncertainties. BD Libertas™ Wearable Injector is a device component intended for drug-device combination products and not subject to FDA 510(k) clearance or separate EU CE mark certification.
* large volume: 2-5 mL or 5-10 mL. - high viscosity: up to 50 cP.
- Design Input Specification for BD Libertas™ Platform [Internal report]. Franklin Lakes, USA: Becton, Dickinson and Company; 2014. Revision: 05
- Design Output Specification for BD Libertas™ Platform [Internal report]. Franklin Lakes, USA: Becton, Dickinson and Company; 2017. Revision: 04
- Woodley, W. D. et al. Clinical Evaluation of an Investigational 5ml Wearable Injector in Healthy Human Subjects. Clin Transl Sci. 2021 May; 14(3):859-869. doi: 10.1111/cts.12946.
- ADDS Libertas Program Preclinical Study List BDTI Parenteral Sciences COE.
- ADDS Libertas Program Clinical Study List BDTI Parenteral Sciences COE.
- Container and device samples available upon request
- BD supports BD Libertas™ Wearable Injector with internal cross-functional expertise in combination product device development and clinical readiness
- BD has validated a filling and assembly process for the BD Libertas™ Wearable Injector (2-5 mL) to support batch clinical supply.3
- Data packages are available to help support combination product development and registration
BD Libertas™ Wearable Injector is a product in development; some statements are forward looking and are subject to a variety of risks and uncertainties. BD Libertas™ Wearable Injector is a device component intended for drug-device combination products and not subject to FDA 510(k) clearance or separate EU CE mark certification.
* large volume: 2-5 mL or 5-10 mL. - high viscosity: up to 50 cP.
- Design Input Specification for BD Libertas™ Platform [Internal report]. Franklin Lakes, USA: Becton, Dickinson and Company; 2014. Revision: 05
- Design Output Specification for BD Libertas™ Platform [Internal report]. Franklin Lakes, USA: Becton, Dickinson and Company; 2017. Revision: 04
- Woodley, W. D. et al. Clinical Evaluation of an Investigational 5ml Wearable Injector in Healthy Human Subjects. Clin Transl Sci. 2021 May; 14(3):859-869. doi: 10.1111/cts.12946.
a BD Libertas™ Wearable Injector is a product in development; some statements are forward looking and are subject to a variety of risks and uncertainties. BD Libertas™ Wearable Injector is a device component intended for drug-device combination products and not subject to FDA 510(k) clearance or separate EU CE mark certification.
b 2-5 mL or 5-10 mL.
c up to 50 cP.
- Design Input Specification for BD Libertas™ Platform [Internal report]. Franklin Lakes, USA: Becton, Dickinson and Company; 2014. Revision: 05
a BD Libertas™ Wearable Injector is a product in development; some statements are forward looking and are subject to a variety of risks and uncertainties. BD Libertas™ Wearable Injector is a device component intended for drug-device combination products and not subject to FDA 510(k) clearance or separate EU CE mark certification.
b 2-5 mL or 5-10 mL.
c up to 50 cP.
- Design Input Specification for BD Libertas™ Platform [Internal report]. Franklin Lakes, USA: Becton, Dickinson and Company; 2014. Revision: 05