Therapeutic and Clinical Areas
Product Brand
Product Family
New Survey Reveals Nearly 3 in 4 Canadian Women Are Delaying Their OB/GYN Exams, Increasing Risks for Cervical Cancer
BD announced the results of a new survey revealing that 74% of women in Canada have delayed a gynecology visit, with 83% wanting more accessible and less invasive cervical cancer testing options, including at-home self-collection for human papillomavirus (HPV) tests.
Women In Canada Can Now Collect Their Own Sample At Home For Cervical Cancer Screening
BD announced today Health Canada approval of the BD Onclarity™ HPV Assay for human papillomavirus (HPV) testing for the use of self-collected vaginal specimens at home.
Mackenzie Health launches first-of-its-kind technology in Canada designed to help reduce IV pump programming medication errors
Mackenzie Health recently launched BD Alaris™ EMR Bidirectional Interoperability, which allows for two-way information flow between an IV pump and a patient’s electronic medical record.
Humber and BD seek to address health human resource challenges
Humber and BD are working together to reshape the landscape of health care workforce development and address the evolving needs of the industry along with health human resources.
Statement on US FDA Safety Communication
BD-Canada is aware of the U.S. FDA issuing Safety Communication stating concern that certain plastic syringes may not provide consistent and adequate quality or performance.
Health Inequity: Tackling the Profound Impact of Chronic Kidney Disease on Indigenous Peoples
The kidney care community advocates for more cost-effective treatment options, which offer a better quality of life and improved outcomes, although several obstacles hinder significant progress in improving kidney failure treatment.
Health Inequity: Bold Action and Collaboration Needed to Eradicate Cervical Cancer in Canada
Cervical cancer is one of the few cancers that is almost completely preventable through systematic screening programs, early detection with HPV testing and vaccination. Our generation holds the incredible potential to seize the opportunity to eradicate cervical cancer within our lifetime.
London’s hospitals are leading the way in clinical microbiology with a world-first installation of state-of-the-art automated laboratory technology
London Health Sciences Centre (LHSC) and St. Joseph’s Heath Care London (St. Joseph’s) are celebrating the installation of a Total Laboratory Automation (TLA) system within the clinical microbiology laboratory.
BD Awards Educational Grant for Point of Care Testing Pilot in Alberta
The program led by 19 to Zero aims to support point-of-care testing needs beyond COVID-19 for urban and rural communities in Alberta through education and diagnosis at clinics.
BD Receives Health Canada Authorization for COVID-19, Influenza A/B, RSV Combination Test
BD announced that it had received Authorization under the Interim Order from Health Canada for a new molecular diagnostic combination test for SARS-CoV-2, Influenza A and B and Respiratory Syncytial Virus (RSV) to help combat a potential “tripledemic” in the upcoming respiratory virus season.
Therapeutic and Clinical Areas
Product Brand
Product Family
Product Category
Product Line
Product Segment
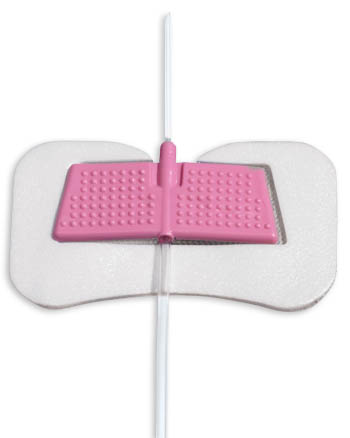


BD offers stabilization devices for latex and silicone catheters. They are available in adult and pediatric sizes.
SKU: vfdssp
-

Preserving sites and protecting veins.
-

Choose pre-filled flush syringes you can rely on
-

The BD Q-Syte™ connector is a high-flow connector that aligns with CDC and INICC design preferences to reduce infections.
-

The Groshong® PICC, Proven performance coupled with proven effectiveness. For years Groshong® PICCs have set the standard in closed-system, vascular access delivery.
-

A validated syringe is critical for patient safety. Your syringe choice may help reduce the potential for serious adverse events during pump infusion.
-

The BD Viper™ LT system provides fully automated, integrated molecular testing on a tabletop analyzer.
-

Fully resorbable implant for soft tissue reconstruction.
-

The EnCor Enspire™ Breast Biopsy System transforms the breast biopsy experience for you, your patient, and your practice.
-

Redefine staff productivity with a fully integrated, automated molecular platform.
-

The Covera™ Vascular Covered Stent builds upon proven technologies from the category leader in AV access.
-

Angioplasty – High Pressure Solutions .035”
-

Designed with advanced filter technology to help prevent movement, tilt and penetration.
Therapeutic and Clinical Areas
Product Brand
- BD Biosciences Inquiries
- Distributor or Wholesale Inquiries
Distributor or Wholesale Inquiries
bdcscanada@bd.com
BD - Canada 2100 Derry Road West Suite 100 Mississauga, ON L5N 0B3 Support
Therapeutic and Clinical Areas
Product Brand
Product Family
Product Category
Product Line
Event Type
Event Format
Therapeutic and Clinical Areas
Product Brand
Product Family
May 01, 2022 - May 01, 2022VirtualThis video and related case study will address medication inventory management in care continuum.
Sep. 24, 2021 - Sep. 24, 2021VirtualThis webinar will focus on lessons learned and best practices of implementing an IV workflow management system highlighting UNC Health’s latest AJHP publication outcomes on the power of remote verification of compounded medications.
Sep. 24, 2021 - Sep. 24, 2021VirtualThis webinar will focus on lessons learned and best practices of implementing an IV workflow management system highlighting UNC Health’s latest AJHP publication outcomes on the power of remote verification of compounded medications.
Sep. 29, 2020 - Sep. 29, 2020Virtual
Sep. 24, 2020 - Sep. 24, 2020VirtualThis webinar will review sterile compounding regulations per USP, ASHP Guidelines, ISMP Guidelines, and The Joint Commission, describe how IV Workflow Management Systems can support USP Compliance through documentation, label requirements, and workflow.
Sep. 24, 2020 - Sep. 24, 2020VirtualThis webinar will review sterile compounding regulations per USP, ASHP Guidelines, ISMP Guidelines, and The Joint Commission, describe how IV Workflow Management Systems can support USP Compliance through documentation, label requirements, and workflow.
- «
- ‹
- 1
- ›
- »